


Healthcare Leadership
Superbugs
Antibiotic Resistance
Brad Spellberg, MD



Scientist
Immunotherapy Research
Author
Healthcare Policy Expert


References
Oral vs. IV Therapy for Osteo in Adults: 11 RCTs (total N = 1,687 patients)
-
Greenberg RN, Tice AD, Marsh PK, et al. Randomized trial of ciprofloxacin compared with other antimicrobial therapy in the treatment of osteomyelitis. Am J Med 1987; 82:266–9.
-
Gentry LO, Rodriguez GG. Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother 1990; 34:40–3.
-
Mader JT, Cantrell JS, Calhoun J. Oral ciprofloxacin compared with standard parenteral antibiotic therapy for chronic osteomyelitis in adults. J Bone Joint Surg Am 1990; 72:104–10.
-
Gentry LO, Rodriguez-Gomez G. Ofloxacin versus parenteral therapy for chronic osteomyelitis. Antimicrob Agents Chemother 1991; 35:538–41.
-
Gomis M, Barberan J, Sanchez B, Khorrami S, Borja J, Garcia-Barbal J. Oral ofloxacin versus parenteral imipenem-cilastatin in the treatment of osteomyelitis. Rev Esp Quimioter 1999; 12:244–9.
-
Schrenzel J, Harbarth S, Schockmel G, et al. A randomized clinical trial to compare fleroxacin-rifampicin with flucloxacillin or vancomycin for the treatment of staphylococcal infection. Clin Infect Dis 2004;39:1285-92.
-
Euba G, Murillo O, Fernandez-Sabe N, et al. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother 2009; 53:2672–6.
-
Li H-K, Rombach I, Zambellas R, Walker S, et al. Oral versus intravenous antibiotics for bone and joint infection. New Eng J Med 2019; 380:425-36.
-
Manning L, Metcalf S, Dymock M, et al. Short- versus standard-course intravenous antibiotics for peri-prosthetic joint infections managed with debridement and implant retention: a randomised pilot trial using a desirability of outcome ranking (DOOR) endpoint. Int J Antimicrob Agents. 2022. 60: 106598. (note, IV arm was 6 wks IV then 6 wks oral)
-
Major Extremity Trauma Research Consortium (METRC). Oral vs Intravenous Antibiotics for Fracture-Related Infections: The POvIV Randomized Clinical Trial. JAMA Surgery. 2025. 160(3):276-284.
-
Juskowich JJ, Thompson JM, Bage SD, et al. Using the Comparing Oral versus Parenteral Antimicrobial Therapy (COPAT) Clinical Trial to Influence Institutional Practice Transformation Towards Earlier Transition to Oral Antibiotics. Clin Infect Dis. 2025. ePub.
Other Prospective IV vs. Oral Study Not Included--Findings--Reason for Exclusion
-
Peacock JE Jr, Pegram PS, Weber SF, Leone PA. Prospective, randomized comparison of sequential intravenous followed by oral ciprofloxacin with intravenous ceftazidime in the treatment of serious infections. Am J Med 1989; 87:185S–90S.--A variety of infection types included, with osteo success in 2/3 oral vs. 3/3 IV--Limitation: only a small number of osteos
9 Other RCTs in Adults Comparing Oral Transitional Therapy in Both Arms (Different Durations or Regimens)
-
Lipsky BA, Baker PD, Landon GC, Fernau R. Antibiotic therapy for diabetic foot infections: comparison of two parenteral-to-oral regimens. Clin Infect Dis. Apr 1997;24(4):643-8.
-
Lipsky BA, Itani K, Norden C. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis. Jan 1 2004;38(1):17-24.
-
Lazaro-Martinez JL, Aragon-Sanchez J, Garcia-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes care. 2014;37(3):789-95. doi:10.2337/dc13-1526
-
Tone A, Nguyen S, Devemy F, et al. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: a multicenter open-label controlled randomized study. Diabetes care. Feb 2015;38(2):302-7. doi:10.2337/dc14-1514
-
Gariani K, Pham TT, Kressmann B, et al. Three versus six weeks of antibiotic therapy for diabetic foot osteomyelitis: A prospective, randomized, non-inferiority pilot trial. Clin Infect Dis. Nov 26 2020;doi:10.1093/cid/ciaa1758
-
Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. Sep 2016;48(3):310-6. doi:10.1016/j.ijantimicag.2016.05.021
-
Benkabouche M, Racloz G, Spechbach H, Lipsky BA, Gaspoz JM, Uckay I. Four versus six weeks of antibiotic therapy for osteoarticular infections after implant removal: a randomized trial. J Antimicrob Chemother. Aug 1 2019;74(8):2394-2399. doi:10.1093/jac/dkz202
-
Bernard L, Arvieux C, Brunschweiler B, et al. Antibiotic Therapy for 6 or 12 Weeks for Prosthetic Joint Infection. N Engl J Med. May 27 2021;384(21):1991-2001. doi:10.1056/NEJMoa2020198
-
Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. Nov 5 2015;385:875-82. doi:10.1016/S0140-6736(14)61233-2
10 Other RCTs or Quasi-Expt in Children Comparing Oral Therapy in Both Arms (all with high success rates)
-
Peltola H, Unkila-Kallio L, Kallio MJ. Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997 99:846-50.
- Peltola H, Vuori-Holopainen E, Kallio MJ, et al. Successful shortening from seven to four days of parenteral beta-lactam treatment for common childhood infections: a prospective and randomized study. Int J Infect Dis. 2001. 5:3-8.
- Jaberi FM, Shahcheraghi GH, Ahadzadeh M. Short-term intravenous antibiotic treatment of acute hematogenous bone and joint infection in children: a prospective randomized trial. J Pediatric Orthop. 2002. 22:317-20.
- Oosterheert JJ, Bonten MJ, Schneider M, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006. 333:1193.
- Peltola H, Pääkkönen M, Kallio P, Kallio M et al. Prospective, randomized trial of 10 days versus 30 days of antimicrobial treatment, including a short-term course of parenteral therapy, for childhood septic arthritis. 2009. 48:1201-10.
- Peltola H, Paakkonen M, Kallio P, Kallio M et al. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatric Infect DIs J. 2010. 29:1123-8.
- Peltola H, Paakkonen M, Kallio P, Kallio M et al. Clindamycin vs. first-generation cephalosporins for acute osteoarticular infections of childhood--a prospective quasi-randomized controlled trial. Clin Micro Infect. 2012. 18:582-9.
- Alcobendas R, Remesal A, Murias S, et al. Outpatients with acute osteoarticular infections had favourable outcomes when they received just oral antibiotics without intravenous antibiotics. Acta Paediatrica 2018; 107:1792-7.
- Bradley JS, Arrieta AC, Digtyar VA, et al. Daptomycin for Pediatric Gram-Positive Acute Hematogenous Osteomyelitis. Pediatric Infect Dis J. 2020. 39:814-23.
- Nielsen AB, Holm M, Lindhard MS, et al. Oral versus intravenous empirical antibiotics in children and
adolescents with uncomplicated bone and joint infections: a nationwide, randomised, controlled, non-inferiority trial in Denmark. Lancet Ped Adolesc Health. 2024. ePub.
References
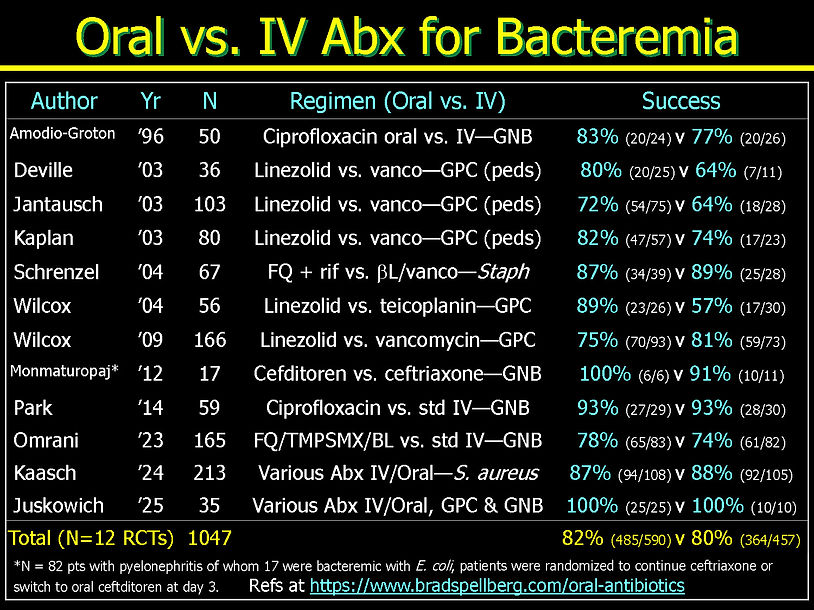
Oral Bacteremia: 12 RCTs (total N = 1,047 patients)
GPC Bacteremia: 8 RCTs*
-
Deville JG, Adler S, Azimi PH, Jantausch BA, et al. Linezolid versus vancomycin in the treatmentof known or suspected resistant Gram-positive infections in neonates. Ped Infect Dis J 2003; 22:S158-S163.
-
Jantausch BA, Deville J, Adler S, Morfin MR, et al. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant Gram-positive bacterial pathogens. Ped Infect Dis J 2003; 22:S164-S171.
-
Kaplan SL, Deville JG, Yogev R, Morfin MR, et al. Linezolid versus vancomycin for treatment of
resistant Gram-positive infections in children. Ped Infect Dis J 2003; 22:677-85. -
Schrenzel J, Harbarth S, Schockmel G, Genne D, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Antimicrob Agents Chemother 1991; 35:538–41.
-
Wilcox M, Nathwani D, Dryden M. Linezolid compared with teicoplanin for the treatment of suspected or proven Gram-positive infections. J Antmicrob Chemother 2004; 53:335–44.
-
Wilcox M, Tack KJ, Bouza E, Herr DL, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 2009; 48:203-12.
-
Kaasch AJ, Lopez-Cortez LE, Rodriguez-Bano J, et al. Efficacy and safety of an early oral switch in low-risk Staphylococcus aureus bloodstream infection (SABATO): an international, open-label, parallel-group, randomised, controlled, non-inferiority trial. Lancet Infect Dis 2024. 24(5):523-534.
-
Juskowich JJ, Thompson JM, Bage SD, et al. Using the Comparing Oral versus Parenteral Antimicrobial Therapy (COPAT) Clinical Trial to Influence Institutional Practice Transformation Towards Earlier Transition to Oral Antibiotics. Clin Infect Dis. 2025. ePub.
GNB Bacteremia: 5 RCTs*
-
Amodio-Groton M, Madu A, Madu CN, et al. Sequential parenteral and oral ciprofloxacin regimens versus parenteral therapy for bacteremia--a pharmacoeconomic analysis. Ann Pharmacol 1996; 30:596–602.
-
Monmontourpaj T, Montakantikul P, Mootsikapun P, Tragulpiankit P. A prospective, randomized, double dummy, placebo-controlled trial of oral cefditoren pivoxil 400 mg once daily as switch therapy after intravenous ceftriaxone in the treatment of acute pyelonephritis. Int J Infect Dis 2012; 16:e843-49.
-
Park TY, Choi JS, Song TJ, Do JH, et al. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci 2014; 59:2790-6.
-
Omrani AS, Abujarir SH, Ben Abid F, et al. Switch to Oral Antibiotics in Gram-negative Bacteraemia; a Randomised, Open-label, Clinical Trial. Clin Microbiol Infect. 2023. 30(4):492-498.
-
Juskowich JJ, Thompson JM, Bage SD, et al. Using the Comparing Oral versus Parenteral Antimicrobial Therapy (COPAT) Clinical Trial to Influence Institutional Practice Transformation Towards Earlier Transition to Oral Antibiotics. Clin Infect Dis. 2025. ePub.
*Juskowich trial had both GPC and GNB organisms for bacteremia, and also had both osteomyelitis and endocarditis patients.
Other RCTs Not Included--Findings--Reason for Exclusion
-
Fass RJ, Plouffe JF, Russell JA. Intravenous/Oral Ciprofloxacin versus Cefepime in the Treatment of Serious Infections. Am J Med 1989; 87(S5):S164-168--Various serious infections--Limitation: only 1 patient with bacteremia and 1 with osteo treated with oral cipro
-
Peacock JE Jr, Pegram PS, Weber SF, Leone PA. Prospective, randomized comparison of sequential intravenous followed by oral ciprofloxacin with intravenous ceftazidime in the treatment of serious infections. Am J Med 1989; 87:185S–90S.--A variety of infection types included, with bacteremia success in 4/5 oral vs. 4/5 IV--Limitation: only a small number of bacteremias
-
Paladino JA, Sperry HE, Backes JM, et al. Clinical and Economic Evaluation of Oral Ciprofloxacin
After an Abbreviated Course of Intravenous Antibiotics. Am J Med 1991; 91:462-470--Various serious infections, N = 99 pts of which 19 had bacteremia, "success" 83% (5/6) for oral vs. 70% (9/13) for IV--Limitation: text suggests micro failures and super infections occurred but cannot tell in which arms or whether they affected bacteremic subpopulation or not -
Bass JW, Steele RW, Wittler RR, et al. Antimicrobial treatment of occult bacteremia: a multicenter cooperative study. The Pediatric infectious disease journal 1993;12:466-73--Children < 3 years with sepsis, 60 bacteremia, randomized to oral Augmentin vs. IM ceftriaxone, few treatment failures at end of therapy are described in either arm--Limitation: it is difficult to discern precise numbers of failures because they are not presented in tables and must be abstracted from text, and there was extensive cross over of oral therapy in the IV arm and IV therapy in the oral arm
-
Mombelli G, Pezzoli R, Pinoja-Lutz G, et al. Oral vs Intravenous Ciprofloxacin in the Initial Emprical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections. Arch Int Med 1999; 159:53-58--53 bacteremic patients among 141 pyelonephritis, no difference in duration of bacteremia oral vs. IV (2.2 d vs. 2.6 d)--Limitation: no description of overall clinical success rates after therapy ended in the bacteremic cohort (but overall cohort and in all subgroups reported, no difference)
-
Geddes A, Thaler M, Schonwald S, et al. Levofloxacin in the Empirical Treatment of Patients with Suspected Bactaraemia/sepsis: comparison with imipenem/cilastatin in an open, randomized trial. J Antimicrob Chemother 1999; 44:499-810--Among 499 pts with sepsis, overall success 77% (184/329) for levofloxacin IV/oral vs. 68% (178/260) imipenem/cilastatin--Limitation: try as I might, I cannot find any description of how many patients were bacteremic or success rates in those cohorts
-
San Pedro GS, Cammarata SK, Oliphant TH, et al. Linezolid versus ceftriaxone/cefpodoxime in patients hospitalized for the treatment of Streptococcus pneumoniae pneumonia. Scand J Infect Dis 2002; 34:720-8--Linezolid was superior in outcome to cephalosporins in patients with bacteremia. The cephalosporin arm also included an oral switch component, so it was not strictly oral vs. IV, it was IV/oral linezolid vs. IV/oral cephalosporin.
-
Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ 2015; 350:h2219--Among 219 pts with S. aureus infection, 91 had bacteremia, overall treatment success not significantly different (but also didn't meet pre-specified non-inferiority definition), bacteremia success (measured at day 7 for some reason, based on clearance of bacteremia and lack of treatment modification) 41% (17/41) v 58% (29/50), 95% CI of difference crosses 0--Limitation: 1) while protocol says oral was allowed, the paper does not indicate that oral was ever used, and TMP-SMX failures were due to prolonged bacteremia, making oral therapy unlikely; 2) strange definition of treatment success, which should be end of therapy or test of cure, not on day 7; 3) TMP-SMX IV (not oral) previously shown to be inferior to vanco for endovascular S. aureus infections from the Markowitz RCT Annals Internal Medicine 1992; 117(5):390-8.

References
Endocarditis: 4 RCTs* and 1 quasi-experimental (total N = 829 patients)
-
Stamboulian D, Bonvehi P, Arevalo C, et al. Antibiotic management of outpatients with
endocarditis due to penicillin-susceptible streptococci. Rev Infect Dis. 1991;13(suppl 2):S160-
S163. -
Heldman AW, Hartert TV, Ray SC, et al. Oral antibiotic treatment of right-sided staphylococcal
endocarditis in injection drug users: prospective randomized comparison with parenteral therapy.
Am J Med. 1996;101(1):68-76. -
Tissot-Dupont H, Gouriet F, Oliver L, et al. High-dose trimethoprim-sulfamethoxazole and
clindamycin for Staphylococcus aureus endocarditis. Int J Antimicrob Agents. 2019;54(2):
143-148. (quasi-experimental, pre-post) -
Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424 & Bundgaard H, Ihlemann N, Gill SU, et al. Long-term outcomes of partial oral treatment of endocarditis. N Engl J Med. 2019;380(14):1373-1374.
-
Juskowich JJ, Thompson JM, Bage SD, et al. Using the Comparing Oral versus Parenteral Antimicrobial Therapy (COPAT) Clinical Trial to Influence Institutional Practice Transformation Towards Earlier Transition to Oral Antibiotics. Clin Infect Dis. 2025. ePub.
*Juskowich trial had osteomyelitis, bacteremia, and endocarditis patients.

References
Pyogenic Liver Abscess: 2 RCTs (total N = 183 patients)
-
Chen Y-N, Chen Y-S, Shin-jung Lee S, et al. A pilot study of oral fleroxacin once daily compared with conventional therapy in patients with pyogenic liver abscess. J Microbiol Immunol Infect 2002;35:179-83.
-
Molton JS, Chan M, Kalimuddin S, et al. Oral vs Intravenous Antibiotics for Patients With Klebsiella pneumoniae Liver Abscess: A Randomized, Controlled Noninferiority Study. Clin Infect Dis. 2020;71(4):952-9.
Other Intra-abdominal Infections: 5 RCTs (total N = 1580 patients)
-
Solomkin JS, Reinhart HH, Dellinger EP, et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intra-abdominal infections. The Intra-Abdominal Infection Study Group. Ann Surg. 1996;223(3):303-15.
-
Cohn S, Lipsett PA, Buchman TG, et al. Comparison of Intravenous/Oral Ciprofloxacin Plus Metronidazole Versus Piperacillin/Tazobactam in the Treatment of Complicated Intraabdominal Infections. Ann Surg. 2000;232(2):254-62.
-
Wacha H, Warren B, Bassaris H, et al. Comparison of Sequential Intravenous/Oral Ciprofloxacin Plus Metronidazole with Intravenous Ceftriaxone Plus Metronidazole for Treatment of Complicated Intra-abdominal Infections. Surg Infect. 2006;7(4):341-54.
-
Fraser JD, Aguayo P, Leys CM, et al. A complete course of intravenous antibiotics vs a combination of intravenous and oral antibiotics for perforated appendicitis in children: a prospective, randomized trial. J Pediatr Surg 2010;45:1198-1202.
-
Arnold MR, Wormer BA, Kao AM, et al. Home intravenous versus oral antibiotics following appendectomy for perforated appendicitis in children: a randomized controlled trial. Pediatr Surg Int 2018;34(12):1257-1268.

References
Complicated Urinary Tract Infections: 5 RCTs (total N = 369 patients)
-
Mombelli G, Pezzoli R, Pinoja-Lutz G, et al. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med. 1999;159:53-8
-
Fang GD, Brennen C, Wagener M, et al. Use of ciprofloxacin versus use of aminoglycosides for therapy of complicated urinary tract infection: prospective, randomized clinical and pharmacokinetic study. Antimicrob Agents Chemother.1991;35:1849-55.
-
Cherubin C, Stilwell S. Norfloxacin versus parenteral therapy in the treatment of complicated urinary tract infections and resistant organisms. Scand J Infect Dis Suppl. 1986;48:32-7
-
Fass RJ, Plouffe JF, Russell JA. Intravenous/oral ciprofloxacin versus ceftazidime in the treatment of serious infections. Am J Med. 1989;87(5A):164S-168S.
-
Sanchez M, Collvinent B, Miro O, et al. Short-term effectiveness of ceftriaxone single dose in the initial treatment of acute uncomplicated pyelonephritis in women. Emerg Med J. A randomised controlled trial. 2002. 19:19–22. (trial found courtesy of Dr. Davie Wong, U. British Columbia)
PNEUMONIA AND SSTI TRIALS COURTESY OF DR. DAVIE WONG, U. BRITISH COLUMBIA
Pneumonia: 1 Meta-Analysis of 12 RCTs of 2,158 Patients Comparing Oral to IV Antibiotics for Treatment of CAP; and 1 post hoc analysis of an RCT showing up front oral therapy worked
-
Teng G-L, Chi J-Y, Zhang H-M, et al. Oral vs. parenteral antibiotic therapy in adult patients with community-acquired pneumonia: a systematic review and meta-analysis of randomized controlled trials. J Global Antimicrobial Res. 2023; 32:88-97.
Overall, oral antibiotic therapy did not affect the incidence of clinical success at the end of treatment (relative risk [RR], 1.01; 95% confidence interval [CI], 0.98–1.05; P = 0.417), clinical success at follow-up (RR, 1.02; 95% CI, 0.98–1.06; P = 0.301), or adverse events (RR, 0.87; 95% CI, 0.56–1.35; P = 0.527). Moreover, oral antibiotic therapy had a beneficial effect on the risk of all-cause mortality (RR, 0.58; 95% CI, 0.35–0.96; P = 0.034).
"We found that oral antibiotic therapy has no significant effect on the incidence of clinical success at the end of treatment, clinical success at follow-up, and adverse events compared with parenteral antibiotic therapy. Moreover, we noted that oral antibiotic therapy was associated with a reduced risk of all-cause mortality compared with parenteral antibiotic therapy."
2. Dinh A, Duran C, Ropers J, et al.; Pneumonia short treatment (PTC) study group. Exclusive oral antibiotic treatment for hospitalized community-acquired pneumonia: a post-hoc analysis of a randomized clinical trial. Clin Microbiol Infect. 2024 Aug;30(8):1020-1028. doi: 10.1016/j.cmi.2024.05.003. Epub 2024 May 9. PMID: 38734138.
N=200 (PO 107, IV 93) PO Amox-clav vs cefotaxime/ceftriaxone/IV amox-clav Success: PO 74% (79/107) vs IV 73% (68/93)
SSTI: 2 RCTs (253 pts)
-
Aboltins CA, Hutchinson AF, Sinnappu RN, et al. Oral versus parenteral antimicrobials for the treatment of cellulitis: a randomized non-inferiority trial. J Antimicrob Chemother. 2015 Feb;70(2):581-6. doi: 10.1093/jac/dku397. Epub 2014 Oct 21. PMID: 25336165.
Success: PO 96% (23/24) vs IV 78% (18/23)
2. Dalen D, Fry A, Campbell SG, Eppler J, Zed PJ. Intravenous cefazolin plus oral probenecid versus oral cephalexin for the treatment of skin and soft tissue infections: a double-blind, non-inferiority, randomised controlled trial. Emerg Med J. 2018 Aug;35(8):492-498. doi: 10.1136/emermed-2017-207420. Epub 2018 Jun 18. PMID: 29914924
Success: PO 96% (92/96) vs IV 94% (93/99)
Possible Serious Bacterial Infections in Babies < 2 mos old: 1 RCT (5253 pts)
-
PSBI Study Group. Switching antibiotic therapy from injectable to oral to optimise the duration of inpatient care for young infants presenting with moderate-mortality-risk signs of possible serious bacterial infection: an open-label, multicountry, randomised controlled trial. Lancet Global Health. 2025. 13:e1903-e1913.
-
PSBI Study Group. Inpatient versus outpatient management of young infants with a single low-mortality-risk sign of possible serious bacterial infection in sub-Saharan Africa and south Asia: an open-label, multicentre, two-arm, randomised controlled trial. Lancet Global Health. 2025. 13:e1892 - e1902.
Pseudomonas in Cystic Fibrosis: 1 RCT (155 pts)
-
Langton Hewer SC, Smyth AR, Brown M, et al. Intravenous versus oral antibiotics for eradication of Pseudomonas aeruginosa in cystic fibrosis (TORPEDO-CF): a randomised controlled trial. Neurology. 2020. 8:975-986.
Neuroborreliosis (CNS Lyme Disease): 3 RCTs (366 pts)
-
Karlsson M, Hammers-Berggren S, Lindquist L, et al. Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis. Neurology. 1994. 44(7):1203-7.
-
Ljøstad U, Skogvoll E, Eikeland R, et al. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: a multicentre, non-inferiority, double-blind, randomised trial. Lancet Neurology. 2008. 7(8):690-5.
-
Kortela E, Kanerva MJ, Puustinen J. Oral Doxycycline Compared to Intravenous Ceftriaxone in the Treatment of Lyme Neuroborreliosis: A Multicenter, Equivalence, Randomized, Open-label Trial. 2021. Clin Infect Dis. 72(8):1323-1331.
Bubonic Plague (yeah, like, seriously people!): 1 RCTs (222 pts)
-
Randremanana V, Raberahona M, Bourner J, et al. Ciprofloxacin versus Aminoglycoside–Ciprofloxacin for Bubonic Plague. New Engl J Medicine. 2025. 393:544-555
Cryptococcal Meningitis in HIV: 1 RCTs (814 pts)
-
Randremanana V, Raberahona M, Bourner J, et al. Ciprofloxacin versus Aminoglycoside–Ciprofloxacin for Bubonic Plague. New Engl J Medicine. 2025. 393:544-555
Honorable Mention: Whipple's Diseases: 1 RCT (All oral vs. some IV and some Oral)
Moos V, Kruger J, Allers K, et al. Oral treatment of Whipple's disease with doxycycline and hydroxychloroquine versus intravenous therapy with ceftriaxone followed by oral trimethoprim–sulfamethoxazole in Germany: a phase 2/3, prospective, open-label, randomised, controlled, non-inferiority trial. Lancet. 2025. ePub.--Not included in the table because predominantly oral in both arms.
Meta-Analysis of Osteomyelitis, Bacteremia, and Endocarditis
Wald-Dickler N, Holtom PD, Phillips MC, Centor RM, Lee RA, Baden R, Spellberg B. Oral Is the New IV--Challenging Decades of Blood and Bone Infection Dogma: A Systematic Review. Am J Med. ePub.
